Insights: A Cerus Leadership Blog
A BRIEF HISTORY OF BLOOD SAFETY AND PATHOGEN REDUCTION TECHNOLOGYDr. Laurence Corash, Chief Scientific Officer at Cerus
(July, 2020) This is part one of a series. Dr. Laurence Corash, chief scientific officer at Cerus, shares his perspective on blood safety and how it has evolved over the last 40 years. He served as chief of the Clinical Hematology Laboratory Service for the Medical Center at the University of California, San Francisco (UCSF) starting in the early 1980s and witnessed, firsthand, the toll and impact that AIDS had on blood transfusion safety in the United States and around the world. Corash explores the history and technological innovation that has been influential in increasing the safety and effectiveness of the world’s blood supply.
A Brief History of Blood Safety and Pathogen Reduction Technology
Annually, over 115 million units of blood are donated worldwide and there are no therapeutic alternatives for patients who need blood1. In the U.S. alone, up to 70% of the population will receive a blood transfusion at some point in their lives2.
Advancements in blood donor screening and testing to ensure a safe blood supply have been influential in lowering the risk for some transfusion-transmitted infections and deaths due to infectious microbial pathogens (viruses, bacteria, and parasites). However, testing before donation is not enough. As years of data show, the implementation of screening tests is a reactive approach that does not eliminate transfusion-transmitted infection due to emerging pathogens previously unrecognized, for example, Zika virus. This is because there is typically a sizable delay between the identification of a new pathogen, understanding the risk of transmission by blood, and the development of a blood donor screening test.
Blood transfusion is a critical, supportive therapy in healthcare, and patients assume that blood is safe when transfused, but this has not always been the case. To understand the fundamentals of transfusion safety, we must first understand the current blood collection and distribution system, and the history and advancements in testing, and the application of pathogen inactivation, or as sometimes called – pathogen reduction, technology to improve blood transfusion safety.
The Blood System in the United States
Blood is collected for transfusion from volunteer healthy donors by non-profit blood banks, for example the American Red Cross blood centers. It can be transfused as whole blood, the blood is separated by blood banks into three components (platelets, plasma, and red cells) before transfusion, to meet specific patient needs based on disease and therapy. Platelets, plasma, and red cells all have different biologic characteristics and require different methods of preparation and storage to retain their therapeutic benefit. Once blood is processed after donation, it is then stored and distributed, in some cases regionally but in other cases nationwide, by blood centers, hospitals and health care providers.
A Brief History of Blood Transfusion Transmitted Infections
When I was a medical intern at Bellevue Hospital in 1969, we had just one test for infectious disease to ensure the safety of blood transfusion. We tested for syphilis, which was only rarely transmitted by transfusion. We were aware that hepatitis, an infection of the liver, occurred commonly after blood transfusion, but we had only surrogate tests to screen blood donors for silent liver disease. These tests were not specific and only helped to screen out some infectious blood donors.
In 1971, nearly a decade before the first known ravages of AIDS, I arrived at the National Institutes of Health (NIH) in Bethesda, Maryland. At that time, a newly developed test, known as the Australia antigen test, was being implemented to prevent the spread of hepatitis B virus (HBV) by blood transfusion. The first test used part of the virus based on the discovery of this viral fragment in the blood of an Australian Aborigine patient. Fortunately, the test for HBV improved rapidly through the 1970s, and by the early 1980s we could identify HVB in most blood donors before transfusion. In parallel, Hepatitis A was recognized for being largely transmitted by food, which meant it was of limited concern for blood transmission (and therefore required no donor testing). However, we still didn’t have a specific test for other blood transfusion-transmitted forms of hepatitis (called non-A, non-B hepatitis), but by 1989, the Hepatitis C virus (HCV) had been identified as the major cause of non-A, non-B hepatitis. Ultimately, the RNA nucleic acid test (NAT) based on the genetic code for HCV became available in 2002, but by then, millions of patients had been infected with HCV. It took 3 decades for us to substantially reduce the risk of HBV and HCV blood transfusion-related hepatitis. This was a significant achievement, but millions of patients acquired transfusion-transmitted hepatitis with a substantial impact on their healthcare over those decades.
The AIDS Epidemic
Prior to the discovery of HCV, the world of blood transfusion safety was shattered by the Human Immunodeficiency Virus (HIV) in the early 1980s, which was proven to be the cause of Acquired Immune Deficiency Syndrome (AIDS). At that time, it was a highly fatal disease. Across the medical community we realized that we had no effective diagnostic tests or therapy.
When I arrived in San Francisco in 1982 to work at the University of California, San Francisco, HIV was in the blood of approximately 1-2% of our blood donors based on modeling and subsequent testing of archived samples3,4,5. Tragically, from the late 1970s when HIV first emerged until 1984, no specific test for HIV existed, which meant there was no way to prevent the infection of patients who needed blood transfusions. Later, the virus was isolated, and tests implemented, but not before thousands of patients were infected by contaminated blood.
Innovations in Blood Safety
After the onset of HIV, other blood transfusion-transmitted viruses emerged to threaten the blood supply; and to make matters worse, the risk of bacterial contamination resulting in fatal sepsis from platelet transfusions was became fully recognized. At the same time, the awareness of arthropod vector born transfusion-transmitted diseases, such as malaria, trypanosomiasis, and babesia increased across the medical ecosystem. As the transmission of these infectious pathogens by transfusion was recognized, new procedures and tests were implemented to address each one.
While each test or new donor screening question improved the safety of blood transfusion, it highlighted a major flaw in the system: testing and donor history were inherently reactive strategies. Even more daunting, these efforts were often not sufficiently effective fast enough when a new infectious pathogen emerged in the blood donor population.
The Future of Blood Supply Safety
Today, multiple new emerging infectious microbial pathogens are recognized as potential threats to humans somewhere in the world each year (Figure 1), and some of these people will be blood donors resulting in possible transfusion-transmitted infection. Since 2004, yellow fever virus, dengue virus, chikungunya virus, Zika virus, Middle Eastern Respiratory (MERS) virus, SARS-CoV-2 virus, Babesia microti, and Trypanosoma cruzi, among others, have impacted the safety and availability of blood transfusion somewhere in the world.
To combat the problems in testing and administering blood donor screening to address the challenge of recurring and new emerging infectious microbes, pathogen reduction offers a new approach to improve transfusion safety. First approved in Europe in 2002, and then in the U.S. by the FDA in 2014, for platelet and plasma components, pathogen reduction treatment (PRT) of blood, offered breakthrough technology to reduce the risk of transfusion-transmitted infections. The goal of this technology, as opposed to testing, was to create a process for blood banks to treat blood components to inactivate, with a single procedure, multiple potential contaminating infectious microbes: viruses, bacteria, and parasites. This technology was based on a basic scientific mechanism of action that in contrast to testing did not require knowledge of the specific characteristics of each new infectious pathogen. As opposed to pathogen reduction, testing of blood to prevent transfusion-transmitted infectious diseases often requires the use of multiple tests for each pathogen (Figure 2), because some tests have limited sensitivity and specificity for detection of pathogens during the “window period,” the time when the level of a pathogen may be below the detection sensitivity of a test. The potential need for multiple assays to address each new threat to the blood supply has called into question the sustainability of the current testing paradigm to ensure prospective safety against emerging threats.
Before FDA approval, pathogen reduction methods did not exist for use in blood banks to treat blood products (platelets, plasma, and red cells). This technology gap motivated the research team at Cerus to develop pathogen reduction methods that could be used in both large and small blood centers for preparation of platelet, plasma, and red cell components based on targeting nucleic acids (DNA and RNA), the genetic code required for viruses, bacteria, and parasites to replicate and cause infection.
Platelets, plasma, and red cells do not require replication of DNA and RNA to deliver therapeutic benefit. Thus, targeted inactivation of pathogen DNA and RNA leaving platelets, plasma, and red cells functional is a strategy that reduces the risk of transfusion-transmitted infection while conserving the therapeutic benefits of blood transfusion. This process is referred to as “pathogen inactivation,” which is performed at blood centers with the same types of plastic containers used to collect and process blood. The treated blood is then sent to hospitals for transfusion. Pathogen inactivation technology is now used in many countries for the preparation of platelet and plasma components. To date, more than seven million blood components treated with this technology have been transfused, and in some countries, it has become the standard of care2. With the emergence of the COVID-19 pandemic, there has been increased interest in the application of PRT to help blood centers prepare for future pandemic threats and secure their blood supply chains
In his next update, Dr. Corash will cover the science behind pathogen inactivation, its 5,000-year-old history dating back to the Egyptians, and why it is relevant to the safety of plasma transfusion as a therapy amidst the COVID-19 pandemic.
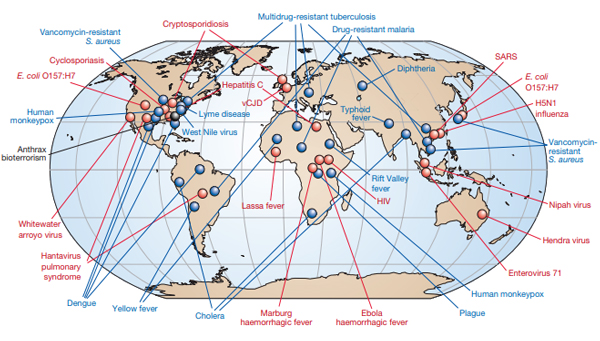
Figure 1. Adopted from Morens DM et al (Morens, Folkers et al. 2004). As of 2004, newly emerging pathogens with potential for blood borne transmission are indicated in red, and previously recognized pathogens with potential for blood borne transmission are indicated in blue.
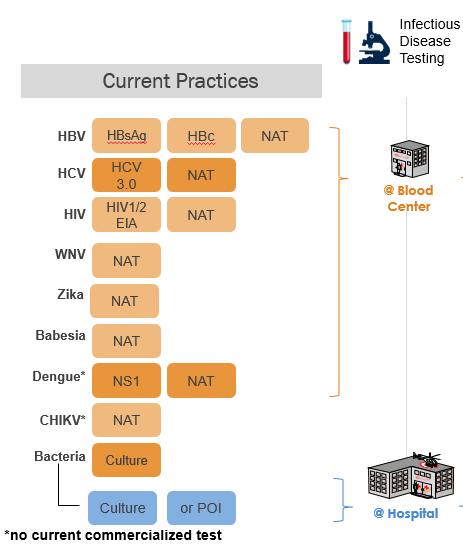
Figure 2. Currently, blood centers use multiple serology and antigen tests to detect HBV, HCV, and HIV, including nucleic acid tests (NAT); and when required additional tests for WNV and Zika. The tests for dengue, and chikungunya are not used in routine practice. Pathogen reduction is FDA approved to replace testing with a single procedure for: bacteria, Babesia, and Zika virus.

About Dr. Laurence Corash
Dr. Corash is the chief scientific officer and co-founder of Cerus. A Professor of Laboratory Medicine at the University of California, San Francisco since 1985, he has served as the Chief of the Hematology Laboratory for the Medical Center at UCSF as well as a consultant to the FDA Advisory Panel for Hematology Devices. In addition to his role as CSO at Cerus, he was previously a member of the U.S. Health and Human Services’ Advisory Committee on Blood Safety and Availability. You can learn more about his work and the formation of Cerus in this video.
Footnotes
World Health Organization, https://www.who.int/news-room/facts-in-pictures/detail/blood-transfusion
UNC Health, https://healthtalk.unchealthcare.org/transfusion/
References
- Busch, M. P., M. J. Young, et al. (1991). “Risk of human immunodeficiency virus (HIV) transmission by blood transfusions before the implementation of HIV-1 antibody screening. The Transfusion Safety Study Group.” Transfusion 31(1): 4-11.
- Jutzi, M., B. Mansouri-Taleghani, et al. (2018). “Nationwide implementation of pathogen inactivation for all platelet concentrates in Switzerland.” Transfusion Medicine and Hemotherapy DOI: 10.1159/000489900.
- Leveton, L. B., H. C. Sox, et al. (1996). “HIV and the blood supply: an analysis of crisis decision making. Executive summary. The Institute of Medicine, National Academy of Sciences Committee to Study HIV Transmission Through Blood and Blood Products.” Transfusion 36: 919-927.
- Morens, D. M., G. K. Folkers, et al. (2004). “The challenge of emerging and re-emerging infectious diseases.” Nature 430(6996): 242-249.
- Perkins, H. A., S. Samson, et al. (1987). “Risk of AIDS for recipients of blood components from donors who subsequently developed AIDS.” Blood 70(5): 1604-1610.